METHODOLOGY
Aim
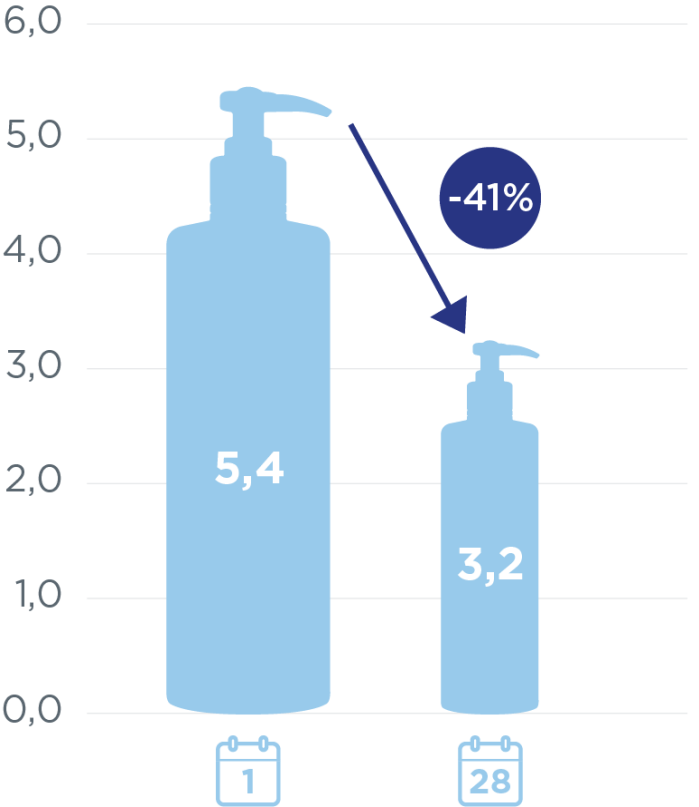
To evaluate the efficacy of an emollient cream (DEXERYL) on xerosis of the feet in diabetic patients.
Study
Prospective, randomised, multicentre, double-blind clinical trial. Contralateral vehicle-controlled (placebo) study.
Population
57 diabetic patients.
Dosage
2 applications daily.
Duration
28 days
Primary evaluation criteria
Assess the severity of xerosis (XAS score) after 28 days of treatment with Dexeryl compared to vehicle-treated feet
Secondary evaluation criteria
Evolution of the XAS score at D14 and the global skin score. Number of feet with cracks, deep cracks, hyperkeratosis, threatening hyperkeratosis and xerosis at D14 and D28. Hydration index measured by corneometry and xerosis by image analysis on D-squame test at D14 and D28. Skin relief at D14 and D28. Subjects' views on treatment
Tolerance
DEXERYL Emollient Cream is safe and well tolerated.