METHODOLOGY
Aim
To demonstrate the effectiveness of DEXERYL Emollient Cream in reducing the severity of ichthyosis.
Study
Phase III, international, multicentre, randomised, controlled, double-blind, parallel-group study: DEXERYL Emollient Cream versus vehicle (placebo).
Population
231 children included with an average age of 8.3 years, suffering from a non-bullous form of ichthyosis: ichthyosis vulgaris (63.2%), X-linked recessive (14.7%), other (22.1%).
Dosage
2 applications daily.
Duration
12 weeks (4-week double-blind period with Dexeryl Emollient Cream or vehicle) followed by an open-label period with all patients treated with Dexeryl Emollient Cream (for 8 weeks).
Primary evaluation criteria
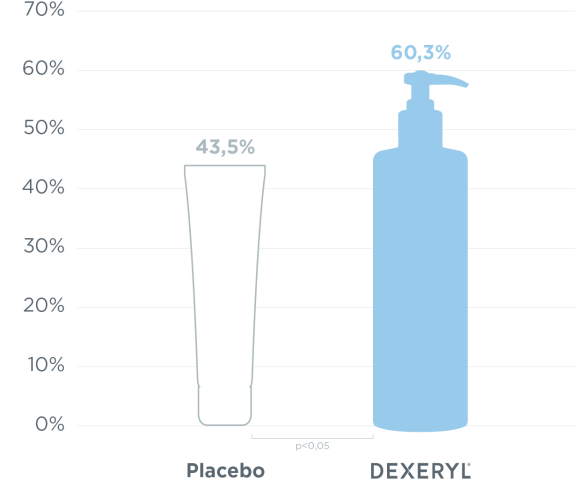
Improvement of ichthyosis by monitoring the evolution of skin xerosis via the SRRC* score: percentage of patients with a 50% reduction in SRRC score at D28.
Secondary evaluation criteria
Pruritus: assessed using a visual analogue scale (VAS). Global assessment (hydration capacity, improvement of perspiration): performed by each investigator and patient.
Tolerance
Twice-daily application of DEXERYL Emollient Cream to the skin of children for 3 months did not induce any systemic or local adverse effects. DEXERYL Emollient Cream has, therefore, proven to be safe and has a good tolerance profile.