METHODOLOGY
Aim
To evaluate the efficacy of glycerin-paraffin cream on uraemic xerosis.
Study
Randomised, double-blind, intra-individual (left versus right), multi-centre clinical study comparing a glycerin-paraffin cream on uraemic xerosis.
Population
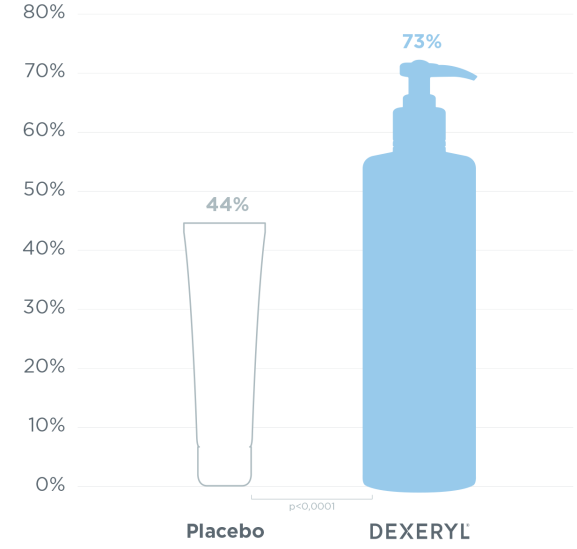
99 randomised patients with moderate to severe uraemic xerosis.
Dosage
2 applications daily.
Duration
8 weeks
Primary evaluation criteria
Treatment response (xerosis score) on each leg, as defined by a reduction of at least two grades in five points from baseline in the clinical score on day 7.
Secondary evaluation criteria
- Patient self-assessment of overall pruritus (visual analogue scale: VAS) and quality of life (SF-12 and DLQI scales).
- Local tolerance, cosmetic acceptability assessed by patients.
- Overall tolerability as assessed by the investigators
Tolerance
DEXERYL Emollient Cream is safe and well-accepted.