METHODOLOGY
Aim
To compare the activity and tolerability of DEXERYL Emollient Cream versus Trolamine in the management of post-radiotherapy erythema.
Study
Phase III, single-centre, randomised (contralateral), double-blind study of Dexeryl Emollient Cream versus Trolamine 0.67% (reference product).
Population
80 randomised adult patients: 70 with breast cancer/10 with ENT cancer.
Dosage
3 applications daily.
Duration
Duration of 12 weeks: 3 applications per day during the first 6 weeks of radiotherapy and then 6 weeks after it has finished.
Primary evaluation criteria
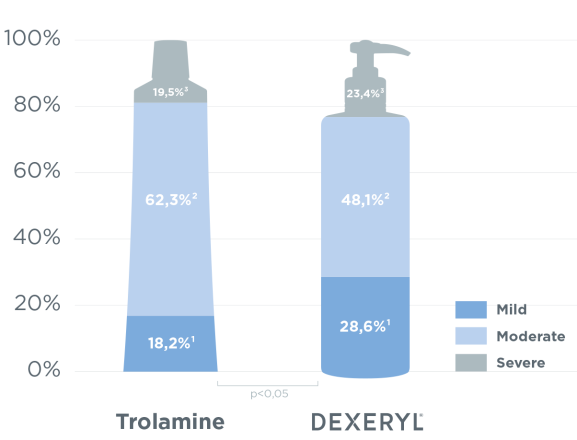
Trophic aspect of treated skin surfaces at D42 (end of radiotherapy) assessed by clinical signs (pain, erythema, exulceration, exudation) using a semi-quantitative scale with 4 scores (0=absent, 1=slight, 2=moderate, 3=severe) and a visual analogue scale assessing the overall trophic aspect of the skin (0: skin lesion - 100: no skin lesion)
Secondary evaluation criteria
- Clinical presentation of treated skin surfaces at D7, D14, D21, D28, D35 and D84 (same parameters as at D42)
- Average time to first skin lesions
- Treatment efficacy assessed by the patient using a visual analogue scale
- Average time of disappearance of skin lesions between D42 and D84
- Acceptability and ease of use of the two treatments evaluated on D7, D42 and D84 according to 3 criteria: ease of application, quality of penetration and sensation after application
- Final evaluation of treatments on D84 according to 5 criteria: treatment efficacy, treatment tolerance, treatment failure in terms of non-compliance, inefficacy and adverse events, preference for one of the treatments and residual hyperpigmentation.
Tolerance
Tolerance is satisfactory for both products.